Glass Transitions and Low-energy Excitations of Vapor-Deposited Simple Molecular Glasses
Yamamuro Group
There remain many unsolved problems in the physical properties of glasses, e.g., glass transitions, boson peaks, beta relaxations, etc. One of the essential approaches to these problems is to study molecular glasses with simple molecular structures such as rare gas and diatomic molecules. This study enables us to make direct comparison with the results of various theoretical and computer simulation studies. The best method to realize simple molecular glasses is a vapor-deposition at low temperature. Its quenching rate is roughly estimated to be more than 107 Ks-1. Recently, we have developed a novel adiabatic calorimeter for vapor-deposited samples. This calorimeter enables us to deposit sample vapor on the inside surface of the cell at ca. 10 K and do in situ heat capacity measurements from 2 K. By using this calorimeter, we have realized the glassy carbon tetrachloride (CCl4) and propene (CH2=CHCH3), both of which have simple molecular structures without any intramolecular conformational change.
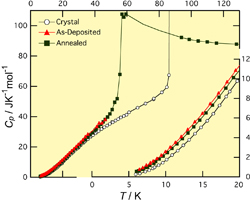
Fig.1. Heat capacities of propene in crystalline, as-deposited glassy and annealed glassy states. The annealed sample was prepared by annealing the as-deposited one around the glass transition temperature 56 K.
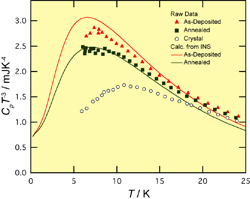
Fig.2. Heat capacities divided by cubic temperature, roughly corresponding to the density of states divided by squared energy. The data calculated from the density of states obtained by inelastic neutron scattering are given for comparison.
On heating the glassy carbon tetrachloride and propene, the former crystallized at 50 K while the latter exhibited a glass transition at 56 K. Figure 1 shows the heat capacities of crystalline and glassy propene. A large jump of heat capacity was observed at the glass transition temperature Tg. The analysis of configurational entropy revealed that the size of the cooperatively rearranging region (CRR) of supercooled propene becomes larger as decreasing temperature and reaches about ten molecules at Tg. Comparing this result with those of other hydrocarbon glasses studied so far, we have found that the CRR size at Tg is correlated with the inverse of molecular mass. Figure 2 shows the heat capacities divided by cubic temperature T3, roughly corresponding to the density of states divided by squared energy. The Debye term will become constant in this plot. The glassy samples exhibit excess heat capacities reflecting the low-energy excitations, usually called boson peaks. The boson peak intensity of the as-deposited sample is larger than that of the annealed one. This result is quite consistent with the previous inelastic neutron scattering data shown by the curves in Fig. 2. These data strongly indicate that the origin of the boson peak is associated with the structural disorder and distortion relaxed by the annealing. Further analyses on the CRR and boson peak are now going on.
References
- Authors
