Interfacial Hydrogen Bonding in Proton-Electron Concerted 2D Organic Bilayer on Au Substrate Probed by Soft X-Ray Spectroscopy
S. Yamamoto, H. S. Kato, and I. Matsuda
Recent developments in the molecular design of organic materials have uncovered a variety of novel functional properties. One of them is the coupling of proton dynamics and electrical conductivity, which can only be achieved in 3D organic crystals. However, reduction of dimensionality to 2D is essential in organic electronics application. In the functional materials group at ISSP, we aim to realize a 2D organic bilayer with a novel “proton-electron” functionality on a solid surface.
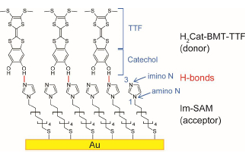
Fig. 1. Schematic structure of organic bilayer of H2Cat-BMT-TTF and Im-SAM on Au. H2Cat-BMT-TTF consists of catechol and tetrathiafulvalene (TTF). Im-SAM has two kinds of N atoms: imino N (N3) and amino N (N1). H-bonds are formed between the imino N atoms in Im-SAM (H+ acceptor layer) and OH groups in H2Cat-BMT-TTF (H+ donor layer).
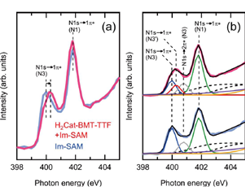
Fig. 2. N K-edge NEXAFS spectra of Im-SAM on Au before and after adsorption of H2Cat-BMT-TTF. (a) Raw data and (b) raw data with peak fitting results.
In this study [1], we prepared and characterized a 2D organic bilayer with “proton-electron” concerted functionality on a solid surface. A “proton-electron” concerted organic molecule, catechol-fused bis(methylthio)tetrathiafulvalene (H2Cat-BMT-TTF), was deposited onto an imidazole-terminated alkanethiolate self-assembled monolayer (Im-SAM) on a Au surface (Fig. 1). In our previous study [2], the OH stretching vibrational modes of H2Cat-BMT-TTF in the IR spectra showed a large red shift and substantial broadening upon adsorption on Im-SAM, indicating that the OH groups of H2Cat-BMT-TTF act as the H+ donor sites. However, the counterpart H+ acceptor sites was not identified due to an overlap of vibrational peaks. Using near edge X-ray absorption fine structure (NEXAFS) spectroscopy, we succeeded in elucidating the nature of H-bonding at the H+ acceptor side (i.e., Im-SAM) because N atoms exist only in the Im-SAM layer.
N K-edge NEXAFS spectra of Im-SAM on Au before and after adsorption of H2Cat-BMT-TTF are shown in Fig. 2. NEXAFS experiments were carried out at soft X-ray beam-line BL07LSU of SPring-8. For Im-SAM on Au, two sharp peaks are observed at 400.0 and 401.8 eV, which are ascribed to the N 1s→1π* transition of the imino N (N3) and amino N (N1) atoms, respectively, of the imidazole ring in Im-SAM. Upon adsorption of H2Cat-BMT-TTF, the π* peak of imino N (N3) shifts from 400.0 to 400.3 eV, while that of amino N (N1) remains at the same energy. The energies of the π* peaks in NEXAFS are sensitive probes of local chemical environments of specific atoms. The energy shift of the π* peak of imino N (N3) suggests that the local chemical environment of imino N is changed by intermolecular H-bonding with H2Cat-BMT-TTF. The H-bonding configuration between H2Cat-BMT-TTF and Im-SAM can be discussed based on the quantitative analysis of the coverage of each molecule. The peak fitting of N K-edge NEXAFS spectra in Fig. 2(b) reveals that the coverage of H-bonded Im-SAM is 0.41 ML. This matches the coverage of H2Cat-BMT-TTF (0.4 ML), which was estimated by X-ray photoelectron spectroscopy in our previous study [2]. This indicates that H2Cat-BMT-TTF and Im-SAM forms H-bonds in one to one fashion (Fig. 1).
In conclusion, the complete picture of H-bonding in the present organic bilayer was obtained; H-bonds form between the imino N atoms (H+ acceptor sites) of Im-SAM and the OH groups (H+ donor sites) of H2Cat-BMT-TTF. The present work is a steady step toward the realization of 2D organic functional materials, and the experimental methods adopted herein will serve as powerful tools for the detection of their functions.
References
- [1] S. Yamamoto, H. S. Kato, A. Ueda, S. Yoshimoto, Y. Hirata, J. Miyawaki, K. Yamamoto, Y. Harada, H. Wadati, H. Mori, J. Yoshinobu, and I. Matsuda, e-J. Surf. Sci. Nanotechnol. 17, 49-55 (2019).
- [2] H. S. Kato, S. Yoshimoto, A. Ueda, S. Yamamoto, Y. Kanematsu, M. Tachikawa, H. Mori, J. Yoshinobu, and I. Matsuda, Langmuir 34, 2189 (2018).
