Hydrogen Bond-promoted Metallic State in a Purely Organic Single-component Conductor
Mori Group
Realization of “purely organic single-component molecular metals” has been one of the long-standing open problems in chemistry, physics, and materials science. As is well known, purely organic materials are normally insulating. Recently, Mori group unveils a new type of purely organic single-component molecular conductors based on a catechol-fused ethylenedithiotetrathiafulvalene, H2Cat-EDT-TTF, and its diselena analogue, H2Cat-EDT-ST, which are designed and synthesized by us [1]. These conductors are the unprecedented single component systems composed of molecular units, H3(Cat-EDT-TTF)2 and H3(Cat-EDT-ST)2, with the highly symmetric intra-unit hydrogen bond. Their electrical conductivity at room temperature is significantly higher than that for the previously reported purely organic single-component systems. Under the moderate physical pressure, moreover, the metallic behavior appeared in the temperature dependence of the electrical resistivity. The higher electrical conductivity observed in our systems is attributed to the hydrogen bond-promoted delocalization of charge carriers, which are generated through the partial oxidation of the H2Cat-EDT-TTF and H2Cat-EDT-ST molecules [2].
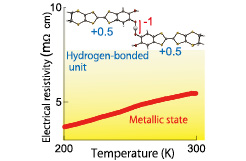
Fig. 1. Structure of the molecular unit and the metallic state in the purely organic single-component conductor. The “purely organic single-component conductor” developed by Mori’s group with the world record conductivity at room temperature (19 Scm-1) is composed of the electrically neutral and symmetric molecular units, where the charge is widely delocalized. The assembled units construct the two-dimensional conducting layers which afford the metallic state under the lowest pressure of around 1 GPa.
A new type of purely organic single component molecular conductors, κ-H3(Cat-EDT-TTF)2 and κ-H3 (Cat-EDT-ST)2, hereinafter described as κ-S and κ-Se, respectively, were obtained as black plate-like crystals by electrochemical oxidation of the corresponding donor molecules, H2Cat-EDT-TTF and H2Cat-EDT-ST, in the presence of the base, 2,2’-bipyridine. The minimal molecular unit, the H3(Cat-EDT-TTF)2 composition (Fig. 1), is established by the formation of an intra-unit hydrogen bond, O..H..O, between the catechol moieties of the donor molecules, where the one hydroxyl proton is deprotonated. The oxygen–oxygen distance in the hydrogen bond, d(O..O), is 2.486(5) Å and 2.509(8) Å at room temperature, and 2.453(5) Å and 2.443(8) Å at 50 K and 30 K for κ-S and κ-Se, respectively, which are much shorter than the length of the normal O–H..O type hydrogen bond, d(O..O) 2.7 ~ 3.0 Å. Because of this strong hydrogen bonding nature, the bonded hydrogen atom is nearly located at the center between two oxygen atoms, in contrast to the asymmetric hydrogen distribution in the normal hydrogen bonds. The minimal molecular units are assembled into the purely organic single component crystal.
The electrical conductivity at room temperature is significantly high, 3.5 and 19 S cm−1 for κ-S and κ-Se, respectively. These values are one or two orders of magnitude higher than the highest reported value, σrt = 10−1 S cm−1, in the purely organic single-component systems, to our best knowledge. As temperature is decreased, the electrical resistivity of these systems exponentially increases with the energy gap Δ/ kB, of 2400 K for κ-S and 1200 K for κ-Se, respectively. The physical pressure is a good tool to change the electronic states by the modulation of the intermolecular interactions. We observed dramatic changes in the temperature variation in the electrical resistivity under pressure for κ-Se. Under the pressure above 1.3 GPa, the electrical resistivity monotonically decreases with reducing temperature down to around 150 K, in striking contrast with the semiconducting behavior at ambient pressure, although the resistive curve turns to increase at low temperature. Thus, the metallic states emerge with the simultaneous suppression of the semiconducting energy gap by the application of the pressure of only 1 GPa. To our knowledge, this is the lowest metallization pressure among the purely organic single-component systems.
Our system demonstrates that the symmetric hydrogen bond constructs the new type of the purely organic single-component molecular conductors which are composed of highly symmetric molecular units. Moreover, we found that the formation of the symmetric hydrogen bond promoted the intermolecular delocalization of the generated carriers, associated with the enhancement of the electrical conductivity. We believe that our new type of molecular conductors with the symmetric intra-unit hydrogen bond will realize the first purely organic single-component molecular metal at ambient pressure. A tetraselenafulvalene (TSF)-type analogue, in which all the sulfur atoms in the TTF part of the present system are replaced with selenium atoms, is a promising candidate for the ambient-pressure metal, because the further enhanced intermolecular interactions are expected.
References
- [1] H. Kamo, A. Ueda, T. Isono, K. Takahashi, and H. Mori, Tetrahedron Lett. 53, 4385 (2012).
- [2] T. Isono, H. Kamo, A. Ueda, K. Takahashi, A. Nakao, R. Kumai, H. Nakao, K. Kobayashi, Y. Murakami, and H. Mori, Nature Commun. 4, 1344 (2013).
