DFT-MD Analysis of TiO2/Solution Interfaces for Photocatalysis and Solar Cell
Y. Tateyama, M. Sumita, and K. Sodeyama
Though a lot of progress has been made in the development of photocatalysis and solar cell, atomistic mechanisms of the chemical reactions at the solid-liquid interfaces have not been fully understood yet because of the experimental and computational difficulty in analyzing such interfacial processes. In order to solve these problems, we have been studying TiO2 electrode/liquid interfaces, associated with photocatalysis (PC) and dye-sensitised solar cell (DSC), on the atomic and electronic scales, by use of quantitative theoretical simulations based on the density functional theory (DFT) with supercomputers.
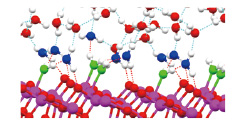
Fig. 1. Equilibrium structure of TiO2 anatase (101)/ liquid water interfaces. Molecular adsorption of water with strong hydrogen bond is observed. The water coverage is around 0.6.
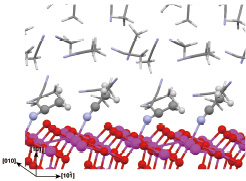
Fig. 2. Equilibrium structure of TiO2 anatase (101) / liquid MeCN interface for efficient DSCs.
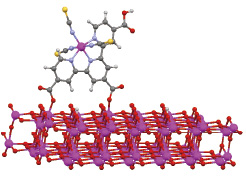
Fig. 3. Schematic picture of black dye adsorption to TiO2 anatase (101) surface for an efficient DSC.
We investigated the TiO2 anatase (101) and (001) interfaces, a most representative PC system, dipped in bulk water on the atomic scale by DFT molecular dynamics (MD) simulations, in order to elucidate the behaviour of water molecules and hydrogen bond (HB) networks at the interfaces [1]. It is demonstrated that the adsorption manners (molecular or dissociative) of water molecules on the vacuum surfaces still hold in the presence of "bulk" water on the interfaces. On the contrary, novel adsorption structures of interfacial water molecules are found as well. We also show explicit atomistic structures of strong and weak HBs at the TiO2/H2O interfaces, which had been proposed experimentally so far. We then suggested a two-layer model for the interfacial water on both surfaces investigated. Our results also give insights into the H2O or OH adsorption coverage at the interfaces and their hydorphobicity and hydrophilicity. To understand the reaction reactivity, we have recently analyzed the equilibrium electronic states of several TiO2/H2O interfaces as well, and found novel microscopic mechanism of the TiO2 photocatalystic reactions.
We have investigated the DSC interfaces as well. TiO2 anatase (101) / liquid acetonitrile (MeCN) interfaces and its water contamination effect were examined by DFT-MD simulations [2]. We clarified the characters of MeCN adsorption and the interfacial MeCN under thermal equilibrium. We also found that unusual way of adsorption is the most stable for the contamination water around the TiO2/MeCN interfaces. The calculated electronic states give an insight into atomistic effect of such water on the dye adsortption and then the durability of DSCs.
We have also examined the adsorption of a Ru N749 dye, so called black dye showing one of the highest conversion efficiency, on the TiO2 anatase (101) surface [3]. Our calculations suggest that this black dye prefers keeping proton energetically, implying that the TiO2 surface is rather acidic. Regarding the photoexcitation spectra, our TDDFT calculations reproduce the red shift of the onset compared to that of the most famous N3 dye.
References
- [1] M. Sumita, C. Hu, and Y. Tateyama, J. Phys. Chem. C 114, 18529 (2010).
- [2] M. Sumita, K. Sodeyama, L. Han, and Y. Tateyama, J. Phys. Chem. C 115, 19849 (2011).
- [3] K. Sodeyama, M. Sumita, C. O'Rourke, U. Terranova, A. Islam, L. Han, D. R. Bowler, and Y. Tateyama, J. Phys. Chem. Lett. 3, 472 (2012).
