Ferroelectric Double Perovskites
I. Ohkubo and R. Takahashi
Various double perovskites with ordered B-site lattices and a general formula of A2M’M’’O6 have recently been synthesized and characterized. The magnetic behavior of these materials is particularly interesting, with half-metallic behavior in Sr2FeMoO6, multiferroicity in Bi2NiMnO6 and Bi2CoMnO6, and semiconducting ferromagnetism in La2NiMnO6 and La2CoMnO6. The purpose of this joint-use project was to perform structural and dielectric characterization on La2NiMnO6 (LNMO) thin films that are semiconducting but become ferromagnetic at an exceptionally high temperature of 280 K. The material is thus a prime candidate for use in room-temperature spintronic device applications. Due to the ordered state of B-site cations with different valence states, such as Ni2+ and Mn4+ in La2NiMnO6, double perovskites are also prime candidates for strong magnetodielectric coupling, where the dielectric behavior can be controlled by applied magnetic fields. Due to the semiconducting behavior of La2NiMnO6, however, accurate dielectric characterization close to the onset of magnetic order is hampered by the large conductivity. Our purpose, therefore, was to use zero-bias pyroelectric characterization to determine if a polar state exists in La2NiMnO6, and to determine if this material might also show a switchable multiferroic response.
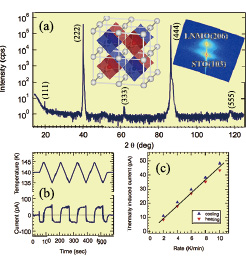
Fig. 1. X-ray diffraction analysis of an ordered perovskite La2NiMnO6 thin film sample. The reciprocal space mapping in the inset shows that the film is coherent on the SrTiO3 substrate. Clear odd-index superlattice peaks due to B-site ordering are visible in the asymmetric diffraction pattern (a). The pyroelectric response of La2NiMnO6 is shown in (b), together with a rate-dependent thermally-induced current plot in (c).
The ordering of the transition metal ions in double perovskites is often incomplete. It is therefore important to determine the structural quality of a crystal before dielectric measurements. The structural analysis was done by conventional x-ray diffraction and reciprocal space mapping analysis, as illustrated for a well-ordered sample in Fig. 1(a). It is clear that the La2NiMnO6 film is coherently grown on the SrTiO3 substrate and the odd-index superlattice peaks characteristic of an ordered phase are clearly visible in an asymmetric diffraction pattern measured for a (001)-oriented thin film sample. The presence of the expected anisotropic polarization-dependent Raman scattering peaks was also verified for the same thin film samples.
The dielectric characterization was done in a planar capacitor geometry. The temperature dependence of the dielectric permittivity, measured at various frequencies, showed a clear relaxor-like behavior between 100 and 200 K. The pyroelectric measurements were done in the same capacitor geometry, using a chopped laser source for periodically heating and cooling the thin film capacitor. For polar materials, the small temperature modulation results in a change of the surface charge due to a temperature dependence of the spontaneous polarization. Since no bias is applied to a sample during the pyroelectric measurement, it is possible to determine the presence of a polar state even for a semiconducting material, where traditional ferroelectric testing would not be possible due to large leak currents. The measurements showed that a polar state does indeed appear in La2NiMnO6 at temperatures below 250 K. The pyroelectric coefficient can be determined from slow temperature sweeps, as illustrated in Fig. 1(b) and looking at the normalized slope of the thermally-induced current as a function of the sweep rate. Analysis of the data in Fig. 1(c) showed that the pyroelectric response in La2NiMnO6 is 34 nC/cm-2K, which is comparable to the well-known lead titanates. This study shows that ordered perovskites, like La2NiMnO6, may be useful not only as spintronic materials, but may also be useful for developing lead-free pyroelectric sensors.
